The Question: Are intracellular targets still intractable for macromolecules ?
There are many validated intracellular targets and novel treatment modalities - but a lack of efficient delivery.
This means:
Host of well characterized intracellular targets are not amenable to small molecule development in a multitude of therapeutic areas.
Endosomal entrapment is a as major limitation to deliver large biomolecules to cytoplasmic/nuclear targets.
Intracellular targets are mostly intractable for large biomolecules, as it requires traversing cellular membranes
- Proteins payloads: typically, do not escape endosome and quickly degrade.
- Oligonucleotides: >99% are trapped in endosomes.
Cancer
- 19.3 million registered new cases worldwide and almost 10.0 million deaths in 2020 [1]
- More than 385,000 children and adolescents (age 0–19) develop cancer each year [2]
- By November 2017, around 2600 clinical trials were conducted on gene therapy, more than 65% of them associated with cancer [3]
- Until August 2019, 22 gene products were approved for the treatment of different disorders, five of them against cancer [4]
- Endosomal escape enhancers augment efficacy and lead to tumor regression [5]
References:
[1] https://doi.org/10.3322/caac.21660,
[2] https://doi.org/10.1016/s1470-2045(17)30186-9,
[3] https://doi.org/10.2147/BTT.S302095,
[4] https://doi.org/10.1016/j.biotechadv.2019.107502,
[5] https://doi.org/10.3390/biomedicines5020014
Hemophilia B
- Hemophilia B is the second most frequent coagulation disorder with a frequency of 1 in 30,000 males [1]
- Current treatment consists of frequent injections of recombinant factor IX (FIX) and has several drawbacks [2]
- Adeno-associated virus-mediated delivery of the FIX coding sequence (gene therapy) showed promising results but still suffers from several limitations [3]
The ENDOSCAPE-based non-viral DNA delivery opens new therapeutic opportunities for gene therapy, and could be applied to many other liver disorders. Endosomal escape enhancers strongly augment transfection efficiency in cellular models.
References:
[1] https://doi.org/10.1016/S0140-6736(03)13405-8,
[2] https://doi.org/10.1186/1750-1172-7-24,
[3] https://doi.org/10.1055/s-0041-1722862
The Solution: Endosomal Escape Enhancer (EEE) for Intracellular Delivery of Macromolecule Compound
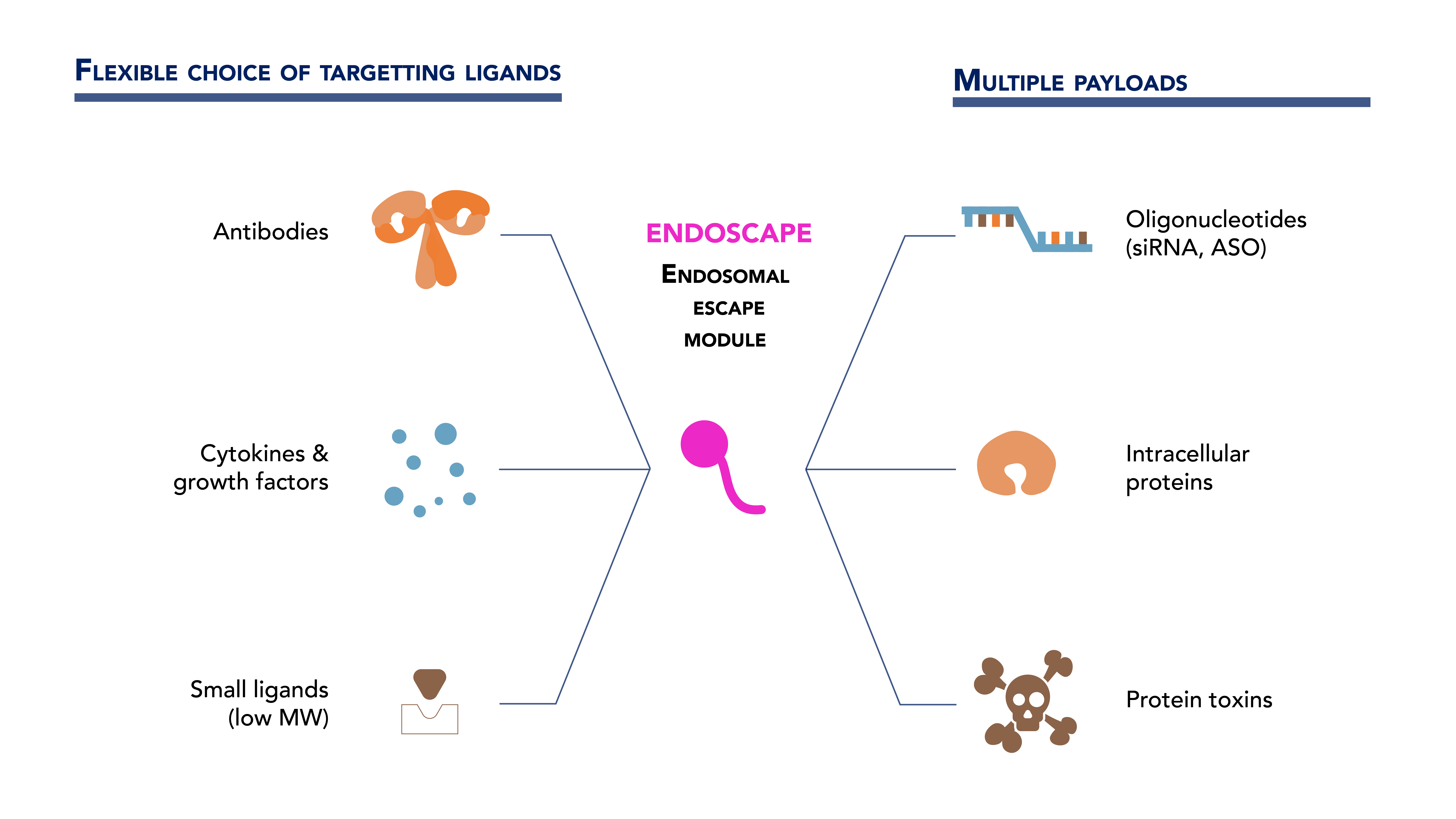
EEE Enables Efficient Payload Delivery From Endosomes
EEE mediates increased release of polymeric drugs in target cells
>> lowering dose + reduced off-target tox
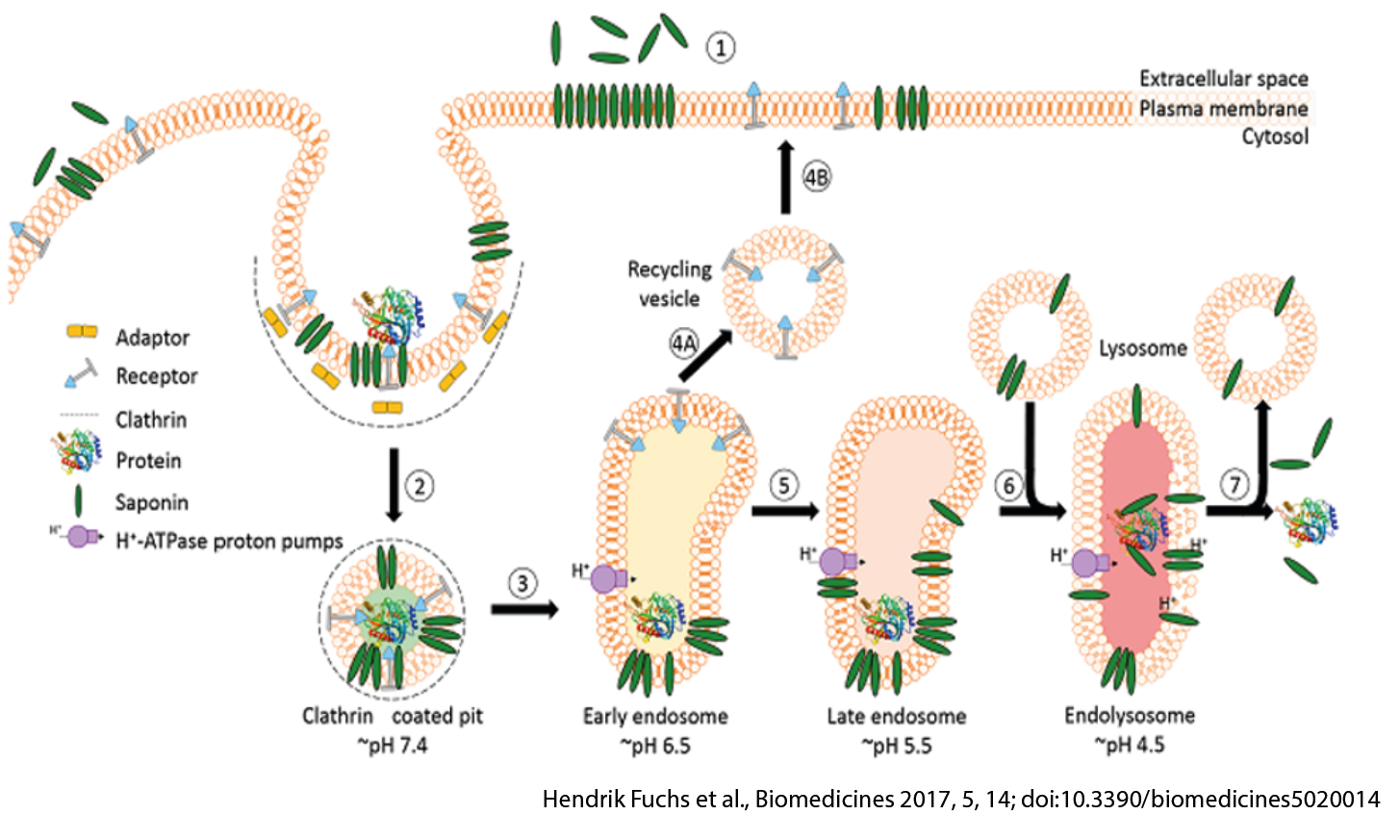
EEE Mode of Action: Endosomal Low pH Driven Activity
EEE mediates efficient endosomal escape from acidified endosomes
Mechanism
- The endosomal escape enhancer activity
- Is dependent on clathrin-mediated uptake of cargo.
- Can be blocked with endosomal acidification inhibitors.
- Proteins that do not route through late endosomes are not amenable to improvement through endosomal escape enhancement.
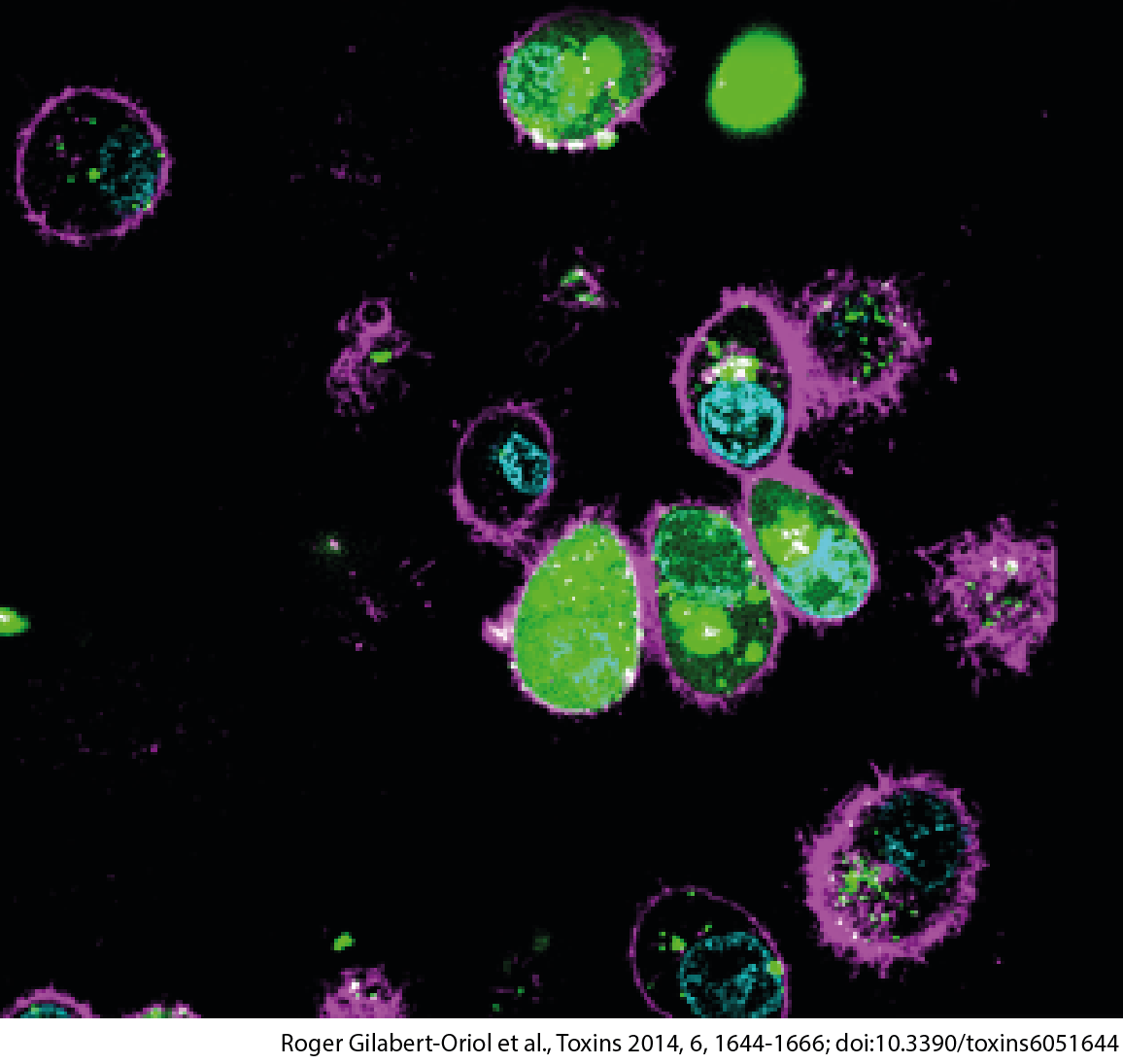
Powered by Evolution: Natural Intracellular Delivery Compounds

- Plants produce protein toxins 10–100 fold more potent than small molecule toxins
- These proteins do not cross endosomal membranes
- Plant metabolites evolved to enhance release of toxins from endosomes – enabling access to cytosol
The ENDOSCAPE Symposium gives answers
- Gene Therapy – Where We Stand Today
- Endosomal Escape – The Strategies
- Carriers of Endosomal Escape Enhancers and Genes
- Endosomal Escape – What Happens?
- GMP Production and Market
- Gene Therapy – Clinical Applications and Future Perspectives